What are X-rays?
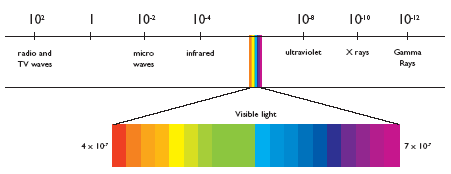
X-rays (RX for simplicity in the rest of the text) are high-energy, short-wavelength electromagnetic radiation. In the radiation spectrum, they are located to the right of light (which itself decomposes from red to violet in its energy spectrum, it's the rainbow!). On the same spectrum, radio waves, microwaves and infrared are located to the left (low energy and longer wavelength).
RX
The reason for using X-rays is quite simple. They have the ability to pass through matter and illuminate certain mineral salts. The combination of these two characteristics allows the production of X-ray films.
X-rays were discovered in 1895 by a German physicist named Roentgen. Not knowing what to call his new rays, he named them after the mathematical constant of the unknown, i.e. "X"! The Roentgen became the unit of measurement for X-ray exposure.
Another interesting feature of RX is the fact that they move in a straight line. They interact with the material crossed being more or less absorbed depending on the thickness and type of tissue encountered. Bone will absorb a lot, lung much less. As a result, the amount of rays that come out of the tissues will vary depending on the material crossed. On the other hand, not being deflected, the place reached on the plate by the ray will always represent the content of the tissue in a straight line upstream.

X-ray of the lungs. The air in the lungs lets more rays through, which can darken the X-ray film more. The heart and bones absorb more, which leaves less to darken the screen.
Production of RX
X-rays are produced in a vacuum tube containing a positive and negative pole (cathode and anode) through which a high voltage current is passed. This causes electrons to move from the cathode to the anode. The resulting movement of electrons is accompanied by the emission of X-rays used for imaging.
Note that, unlike radioactivity, X-ray emission only occurs when a high voltage is applied. As soon as the button is released, there is no more radiation!
Much like the filament of an incandescent light bulb, X-ray tubes have a useful life and must be replaced periodically when the efficiency of the X-ray generation process decreases. This, together with the electricity required, represents a significant portion of the operating expenses.
Interactions in matter
Once emitted, RXs therefore travel in a straight line towards a target. Their properties of interactions with matter determine their use.
Let's do it in reverse. Let's first see what happens at the level of the film reached (we will then see what happens in the human tissues crossed).
- Interactions in X-ray film
For a long time, X-ray films were made of a plastic support containing silver salts. The absorption of X-rays by these salts triggers a physicochemical reaction that modifies their properties. All that remained was to pass these films through a developing device (a process similar to photographic films, before the digital era) to obtain a X-ray image.
Today, silver nitrate films have been replaced by sensors containing various substances that have the property of emitting light when stimulated by X-rays.
- Phosphorus in the case of CR (computed radiography ). In CR, the image is latent and must be scanned by a laser to be visualized. This was a transitional step that still has some applications but is used less and less. It was faster but we did better later.
- Silicon, cesium or even gadolinium in the case of DR (digital radiography). These rare earths emit light that is captured directly by a CCD camera which produces an instantaneous image.
A quick word on the benefits of digital imaging. Beyond acquisition speed, there are other gains to consider.
- Interactions in the patient
So let's back up a bit. Before the X-rays reach the film, they have to pass through the patient. So what happens there?
X-rays are ionizing. That is, they have the ability to modify the structure of the atoms encountered by moving electrons, making the atoms positively or negatively charged (creation of ions).